
Implant Technologies
OsteoSync™ Ti
Different by Design
Experience the benefits of OsteoSync TI, a porous ingrowth material that offers best-in-class bone ingrowth without the risks or high costs associated with other materials. Perfect for the ASC market, it caters to younger, more active patients who have higher expectations for their procedures and where price sensitivity is higher. Clinically proven, with over 250,000 devices implanted since 2014, OsteoSync TI is a trusted choice.

Structural Features for Enhanced Ingrowth
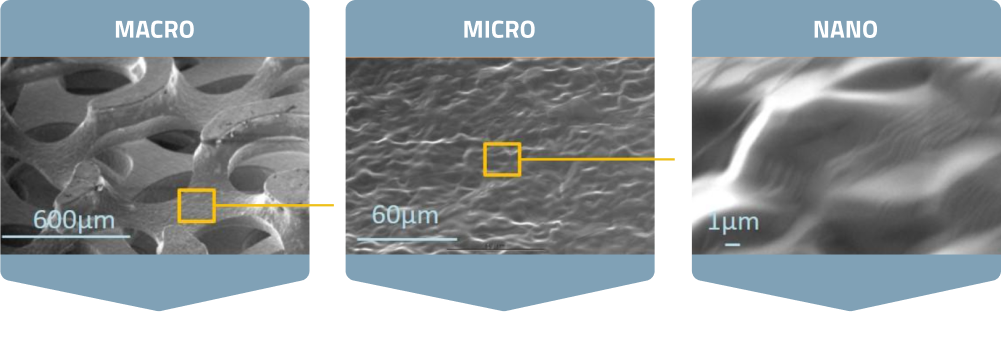
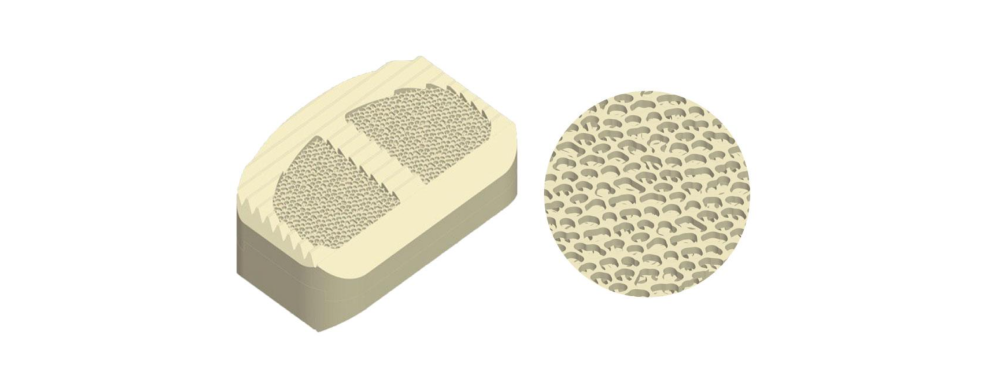
OsteoSync Ti is a titanium material with macro, micro and nano-scale features that promote early and aggressive bone ingrowth. With an average porosity of 60%, an average pore size of 523µm and an interconnected pore size of 229µm, it meets the ideal macro-scale criteria for significant bone ingrowth.
The nano-scale structures, combined with micro and submicron-scale roughness, enhance osteoblast differentiation and local factor production, leading to faster healing times and improved implant osseointegration in-vivo.
Exceptional Performance
- Rapid Bone Ingrowth: Demonstrates significant bone volume ingrowth in both dynamic and static animal models in as few as 6 weeks.
- Superior Strength: Exhibits 2 to 5 times greater bone volume ingrowth compared to 3D-printed materials and nearly double the shear strength of titanium plasma spray at the 5-week follow-up.

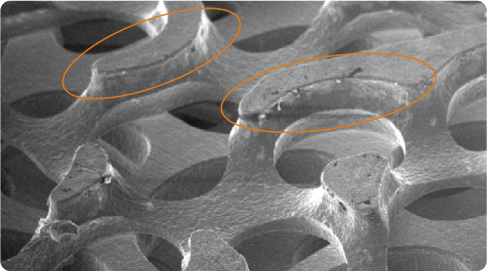
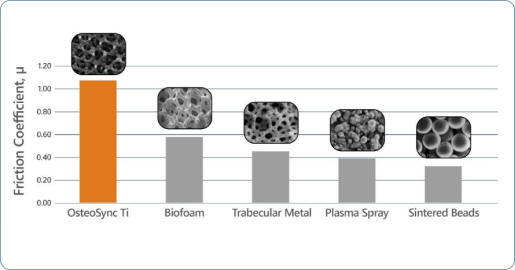
High Coefficient of Friction
The matrix’s longer edges provide a higher coefficient of friction for improved initial implant stability, essential for long-term bone ingrowth.
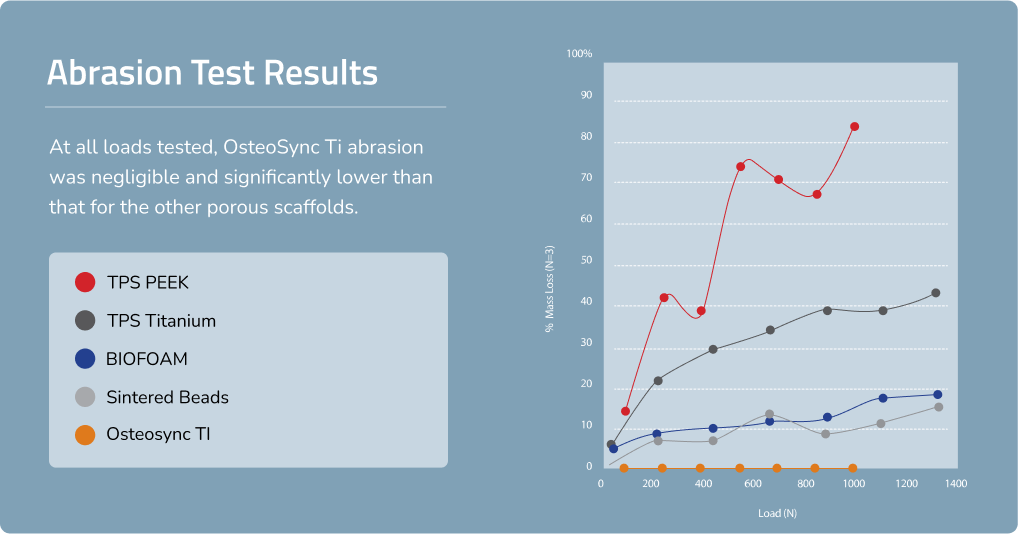
High Resistance to Abrasion
The sheet-like nature of OsteoSync Ti ensures minimal debris generation during implant insertion, with testing showing negligible mass loss after 10 simulated cycles at all loads tested.
True Platform Technology
OsteoSync Ti has proven high attachment strength to various metals (Ti, CoCr) and polymers (PEEK, UHMWPE, PCU). With a nominal thickness of 1mm, it can be made as thin as 0.5mm or as thick as needed, enabling fully cementless implants to improve OR efficiency and versatility in orthopedic applications.
Choose OsteoSync Ti for its proven performance and adaptability. Let us support your success with our innovative technology designed to meet your needs and exceed your expectations.

OsteoSync™ Ti Flex*†
OsteoSync Ti Flex is designed to address your needs for minimizing stress shielding and interbody fusion device subsidence. This innovative variable stiffness ingrowth technology allows surgeons to select an interbody device with stiffness that closely matches the bone it is placed against. Fully porous to maximize the fusion mass opportunity, OsteoSync Ti Flex is intended to provide optimal patient outcomes.

*Technology under development. Patents filed. | †Technology not currently cleared with the FDA.
Join the forefront of orthopedic innovation with OsteoSync Ti Flex. Contact us today to learn how this technology can enhance patient outcomes.
OsteoSync™ Rx*†
OsteoSync Rx is a breakthrough implant designed to deliver various treatments through channels and perforations directly to the implant-tissue interface. Tailored to address specific clinical conditions as determined by the physician, this technology offers a personalized approach to patient care. Potential conditions treated include infection, osteosarcoma, osteoporosis or a combination thereof.
Interbody Fusion Device Concept
Featuring a contained graft area and cannulated instruments for in-situ graft delivery, this concept enhances OR efficiency and offers better fixation by suspending bone graft throughout the matrix. Accommodating a wide range of graft options, it also represents a future platform for therapeutic delivery.
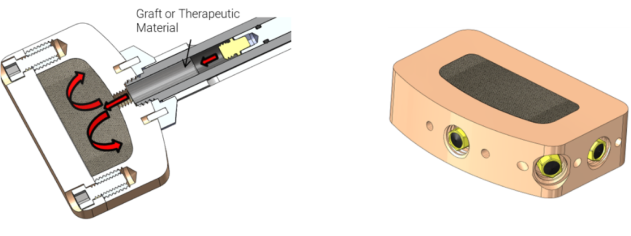
Knee Component Concept
Designed to prevent post-op infection by delivering antibiotics at the time of surgery and shortly afterward, it also treats late-onset active infections with antibiotics via a needle passing through soft tissues to the injection port.
*Technology under development. Patents filed. †Technology not currently cleared with the FDA.
Elevate your treatment capabilities with OsteoSync Rx. Contact us today to discover how this novel implant can meet the specific needs of your patients and enhance clinical outcomes.
OsteoSync™ PEEK
OsteoSync PEEK offers a highly porous Polyether ether ketone (PEEK) polymer scaffold material to improve implant fixation and clinical ease of use, with wide applications in orthopedics.
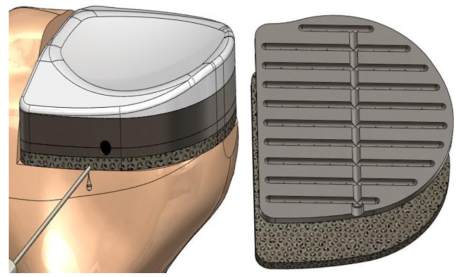
Interbody Fusion Device Concept
OsteoSync PEEK material at the center of the device facilitates bone ingrowth and spinal fusion without autologous bone harvesting, saving valuable OR time and reducing potential co-morbidities. It also potentially eliminates the need for allograft or bone graft substitutes, thus reducing healthcare costs.
Implant Augment Concept
OsteoSync PEEK augments attached to TKR implants provide initial bone support and long-term stability similar to solid metal augments. During future revision procedures, a bone saw can cut through the OsteoSync PEEK material, leaving more bone and providing a solid foundation for implant support, potentially improving revision outcomes.
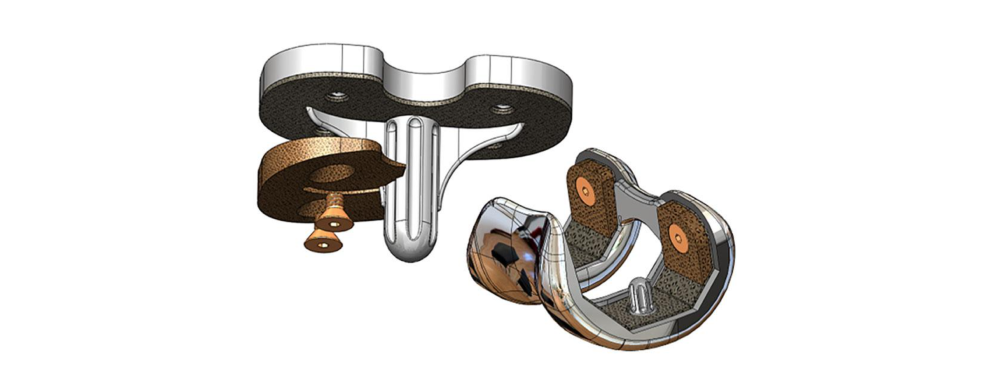
Soft-Tissue Suture Anchor and Interference Screw Concepts
Using OsteoSync PEEK, this concept aims to improve long-term implant stability with bone integration into the implant.
Explore OsteoSync technologies to provide your patients with cutting-edge solutions designed for better outcomes and clinical efficiency.

